Author | Pan Xiaoben
Researcher, Peking University People's Hospital, Peking University Institute of Liver Diseases
July 28, 2017 is the seventh World Hepatitis Day with the theme "eliminate hepatitis."
If the time was returned to the day of 1925, in Brooklyn, New York, a family of lawyers welcomed the birth of a boy named Baruch Samuel Blumberg. In Hebrew, Baruch is blessed, and Samuel is the Hebrew prophet. Blumberg apparently accepted the blessing. He discovered the hepatitis B virus at the age of 38. At the age of 51, he won the Nobel Prize in Physiology or Medicine for this discovery.
Epidemiological surveys show that up to one-third of the world's population has been infected with the virus, and 350 million of them have developed chronic infections. It is the discovery of hepatitis B virus that kicks off the pathogenesis diagnosis, treatment and prevention of viral hepatitis. To commemorate Blumberg's outstanding contribution to the field of hepatitis, in 2010, the World Health Organization (WHO) decided to start his birthday as World Hepatitis Day in 2011. The theme slogan of the first World Hepatitis Day is: This is hepatitis...
Hepatitis B virus
Human understanding of hepatitis is actually a long history. As early as 2000 BC, there was a first record of the epidemic of jaundice, which has been considered contagious because it often occurs in areas with high population density and poor sanitation. From 460 BC to 375 BC, Hippocrates, the originator of Western medicine, mentioned "epidemic jaundice" in "Medicine Diseases". In China's "Yellow Emperor's Canon", there are also records of jaundice. In ancient times, the descriptions of these jaundice were mostly viruses. Clinical manifestations of sexual hepatitis.
In 1908, S. McDonald speculated that there might be a virus that causes liver disease, but how it spread is a mystery. In 1947, British doctor FO MacCullum, who is engaged in liver disease research, suggested that there may be two kinds of viruses: one that spreads hepatitis A through the faecal-oral route, called hepatitis A; the other that spreads through the blood, called hepatitis B. Blumberg, who served as a US Navy officer in World War II, had just retired and was studying for a doctorate in medicine at Columbia University School of Medicine. But Blumberg's interest is not in hepatitis. After a few years of residency at the Columbia University Presbyterian Medical Center, he moved to Oxford to study for a Ph.D. in biochemistry. His research interest at the time was: Why are some people prone to certain diseases in the same environment, and some people will not? To answer this question, he collected blood samples from around the world to analyze differences in protein content in the blood, trying to study the association between protein diversity and disease susceptibility. For example, the application of the new technology at that time - starch gel electrophoresis - to observe the nuances of human and animal serum protein migration, and then to find the polymorphism and distribution characteristics of the protein. He did have several new discoveries, but there was no big breakthrough.
Blumberg and colleagues began to switch ideas: if many serum proteins have polymorphic variations, then this variation may be antigenic. For patients who have received multiple transfusions, if they do not have polymorphic variations themselves, it is possible to produce antibodies.
According to this idea, in 1960 they began to introduce the new technology immunoelectrophoresis at that time, which is a method for analyzing the composition of antigen by combining agar electrophoresis and two-way agar diffusion. Their hypothesis was quickly supported by experimental results: a patient who had undergone multiple blood transfusions, whose serum reacted with the serum of multiple people in a specific population. Although it was later confirmed that this was only due to the polymorphism of serum low-density lipoprotein in the population, this shows that this research idea may bring some interesting findings.
The facts did not disappoint them. In 1963, Blumberg found antibodies that were clearly unrelated to low-density lipoprotein polymorphism in a number of transfused hemophiliacs. Because it initially reacts with an Australian sera, it is called "Australian". The population study found that "Australian" is rare among ordinary Americans, but it is more common in leukemia patients, so Blumberg initially speculated that "Australian" may be a risk factor for leukemia. However, long-term clinical observation of leukemia patients found that one patient who did not initially detect "Australian anti-", developed liver inflammation while detecting "Australian anti-". The discovery began to point to "Australian" and may be related to clinical hepatitis. More research has subsequently shown that "Australian" is a component of this hepatitis virus.
In 1970, British virologist David Dane observed the complete particle of the virus (later named Dane's particle) by electron microscopy. Because "Australian" is closely related to post-transfusion hepatitis, it conforms to the concept of hepatitis B proposed by MacCullum. Since the 1970s, this hepatitis virus associated with "Australian" has been named hepatitis B virus (HBV).
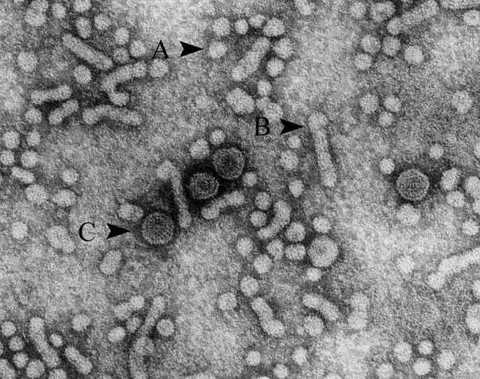 |
| Hepatitis B virus. A and B are subviral particles composed of surface antigen proteins, and C is intact hepatitis B virus Dane's particles. |
Development of hepatitis B vaccine
After the hepatitis B virus was discovered, the vaccine research was put on the agenda. In the serum of hepatitis B infected people, there are a large number of small spherical particles composed only of viral surface antigen proteins, and Blumberg realizes that these non-infectious viral proteins can be used to prepare hepatitis B vaccine, and a preparation process has been developed. In 1975, he transferred the patent to the pharmaceutical company Merck to produce a commercial vaccine.
After rigorous clinical trials, the FDA approved the clinical application of blood-borne hepatitis B vaccine in 1981. In the mid-1980s, due to the development of genetic recombination technology, genetically engineered hepatitis B vaccine was widely promoted and used because of its safer and more stable quality, and the blood-borne vaccine was withdrawn from the historical stage.
The use of hepatitis B vaccine has greatly reduced the prevalence of hepatitis B. In Taiwan, where vaccines were used earlier, the prevalence of hepatitis B fell from 10.9% in 1984 to 1.5% in 1989, while Japan fell from 3% to 0.3%. At present, the number of people with chronic hepatitis B infection has decreased from 350 million to about 250 million. In 2015, the prevalence of chronic hepatitis B infection in children under five years old dropped from 4.7% before vaccination to 1.3%.
Hepatitis C virus discovery
Since hepatitis B can be transmitted through blood transfusions, US legislation in 1972 requires all blood donors to have blood screening in advance. By the late 1970s, with the emergence of more sensitive detection methods, developed countries generally required blood donors to test hepatitis B virus, which greatly reduced the viral hepatitis caused by blood transfusion. But doctors found that despite strict blood screening for blood donors, many people still get hepatitis after blood transfusion. Therefore, doctors referred to this type of hepatitis caused by an unknown virus after transfusion as "non-A, non-B hepatitis."
In search of this hepatitis virus, scientists have examined the blood of these hepatitis patients. Although electron microscopy, artificial culture, and immunology have been used to find the virus, the "non-A, non-B hepatitis" virus has not been found.
In 1983, Chiron of the United States decided to fund a large-scale research program to solve this problem. The Centers for Disease Control and Prevention and Chiron's many scientists have invested in this research project. The scientists first let the chimpanzee infect non-A, non-B hepatitis, and then provide the chimpanzee infection serum to Chiron for analysis. Until 1989, Michael Houghton et al. finally cloned the virus using molecular biology techniques. Since this is the third "hepatophila virus" that has been found to specifically infect human liver, it has been named hepatitis C virus. Subsequent clinical investigations confirmed that 80% to 90% of non-A, non-B hepatitis is caused by hepatitis C virus infection. Different from the past using electron microscopy, artificial culture or immunological methods, this is the first time that scientists have applied molecular biology techniques to directly discover viruses and clone gene sequences, opening up a new field for the discovery of infectious pathogens. Great progress. After 1990, with the promotion of sensitive and efficient hepatitis C virus blood test methods, viral hepatitis in developed countries has been reduced to one in 100,000 by blood transfusion.
Development of hepatitis C antiviral drugs
Compared with hepatitis B, hepatitis C infection is more harmful. The chronicity of hepatitis B infection mainly occurs in infants and young children, and more than 90% of adult infections are recessive or acute infections. However, the chronicization rate after hepatitis C infection is 60% to 85%. Slow hepatitis C can develop into end-stage liver disease such as cirrhosis and liver cancer unless effective antiviral therapy is performed. Another difficulty with hepatitis C is that unlike the hepatitis B virus, the surface protein is highly conserved and easy to develop vaccines. The hepatitis C virus outer membrane protein sequence is highly variable, and vaccine development is difficult. This makes the development of antiviral drugs for hepatitis C particularly urgent.
For the development of antiviral drugs, an efficient and stable drug research platform is essential. Ralf FW Bartenschlager of Heidelberg University in Germany and Charles M. Rice of Rockefeller University in the United States finally established a replication and infection model of hepatitis C virus in laboratory culture cells through in-depth study of the mechanism of replication and infection of hepatitis C virus. In 2007, Arbutus biopharmaceutical company Michael J. Sofia screened a new drug, Sophibuvir, which was effective in inhibiting hepatitis C virus replication, and was approved by the FDA in 2013, which greatly revolutionized the treatment of hepatitis C. In March, the three scientists also won the "Lasque Award", which is known as the "Nobel Prize Vane".
Since then, with the more efficient and less harmful side effects of hepatitis C antiviral drugs, the cure rate of hepatitis C has been nearly 100%. For the foreseeable future, hepatitis C will become a "rare disease", which will also be the first viral infection in human history to be completely cured by drugs.
New anti-hepatitis B drug development accelerated
Compared with the rapid development of hepatitis C drug research, hepatitis B drugs appear to be inferior.
Since 1998, lamivudine, which strongly inhibits the activity of hepatitis B virus polymerase, has been approved for the treatment of chronic hepatitis B. Since then, the FDA has also approved a series of similar drugs that inhibit the virus and are less prone to drug-resistant mutations. Although these drugs can strongly inhibit hepatitis B virus replication, less than one-third of people can achieve the effect of continuously inhibiting viral replication after stopping the drug. Most people will rebound after stopping the drug.
The characteristics of the life cycle of hepatitis B virus make it difficult to completely eliminate the viral genome than hepatitis C: Hepatitis B virus is a double-stranded DNA virus, and its viral genome can be hidden in the nucleus of liver cells to form a microchromosomal structure. Hepatitis C virus is a single-stranded RNA virus whose viral replication process is only present in the cytoplasm. These characteristics make the hepatitis B virus genome more stable and more difficult to attack by immune molecules. Another difficulty is the lack of efficient and practical hepatitis B virus cell infection models for many years, which also limits the development of anti-hepatitis B drugs.
However, a remarkable achievement in recent years is that Li Wenhui, a researcher at the Beijing Institute of Life Sciences, identified key receptors for hepatitis B virus entry into hepatocytes, and based on this, established a model of hepatitis B virus cell infection, which will accelerate the development of new anti-hepatitis B drugs. In addition, with the solution of the hepatitis C treatment drug problem, more scientific research forces have invested in the research and development of hepatitis B drugs. In recent years, a series of new target drugs have entered the clinical trial stage, and the elimination of hepatitis B has also been dawned.
Other hepatitis virus
In addition to hepatitis B and C viruses, as the name implies, there should obviously be other hepatitis viruses.
In 1973, a team of scientists from the National Institutes of Health, led by Steven Feinstone, confirmed the virus that caused hepatitis A. The virus came from a stool sample from a prisoner's volunteer. Hepatitis A is transmitted through the “fecal mouth†route. A disturbing fact is: "If you have hepatitis A, you may have eaten some part of the stool of others." But it is comforting that hepatitis A is a self-healing disease that generally does not cause serious consequences. An example is Shanghai in 1988. Due to the consumption of hairy mites contaminated with hepatitis A virus, there were 340,000 cases of hepatitis A pandemic, a history of the history of hepatitis A in the world. Finally, a total of 31 patients died, and these death patients themselves have some chronic diseases, such as chronic bronchitis, emphysema, and chronic hepatitis B, cirrhosis and so on. In 1981, microbiologist Maurice Hilleman developed a hepatitis A vaccine.
In 1978, the Italian gastroenterologist Mario Rizzetto and the molecular virologist John Gerin of Georgetown University in the United States jointly discovered the hepatitis D virus, but this rare virus is a defective virus and cannot be infected independently, depending on the hepatitis B virus. survive.
In 1983, Mikhail Balayan of the Moscow Institute of Polio and Viral Encephalitis discovered the hepatitis E virus. Like hepatitis A, hepatitis E is also transmitted by contaminated water or food through the digestive tract and can cause local epidemics. In 2012, Xiamen University professor Xia Ning Shao developed the world's first hepatitis E vaccine.
Compared with hepatitis B and hepatitis C, which can cause chronic liver disease, the prevalence and pathogenicity of hepatitis A and hepatitis E are relatively small. Therefore, although there are already vaccines, universal vaccination is not provided to the public, and food hygiene is emphasized to prevent illness from entering the mouth.
Prevention and treatment of viral hepatitis in China
China has always been an important link in the global viral hepatitis prevention and control map.
China was once a high-risk area for chronic hepatitis B, with an infection rate of 10%, accounting for one-third of the globally infected population. In the prevention and treatment of viral hepatitis in China, one of the pioneers to be mentioned is Professor Tao Qimin from the Institute of Liver Diseases, Peking University (formerly the Hepatology Department of Beijing Medical College). She was the first to introduce the detection method of hepatitis B virus in the early 1970s and promote it nationwide. In 1975, she and her foreign countries began the purification of hepatitis B surface antigen protein and vaccine preparation research. At that time, just after the end of the domestic Cultural Revolution, in the case of difficult scientific research conditions and no animal models available, in order to verify the effectiveness and safety of the vaccine developed, Professor Tao Qimin injected himself with the first blood source of China in a fearless spirit. Sexual vaccine. She has achieved success! Since then, the blood-borne hepatitis B vaccine has been gradually promoted nationwide until the 1988 genetic engineering hepatitis B vaccine was put into use in China. Professor Tao won the first prize of National Science and Technology Progress for this purpose. After the discovery of hepatitis C, Tao Qimin also carried out the research and development of domestically produced hepatitis C detection reagents, which is the first step to reduce the spread of hepatitis C after transfusion in China.
In addition to the role of vaccines and drugs, the development and implementation of infectious diseases also play a key role in the prevention and treatment of infectious diseases. In 2002, the Chinese government decided to include hepatitis B vaccine in children's program immunization, and since 2005, all newborns have been vaccinated free of hepatitis B vaccine. With the promotion of hepatitis B immunization prevention, the prevalence of chronic hepatitis B and the mode of transmission of hepatitis B in China have changed significantly, and have been reduced from high epidemic areas to middle epidemic areas. In 2012, WHO sent a congratulatory letter to congratulate China on the successful use of hepatitis B vaccine to control hepatitis B, and regard China's success as a model for the region and the world. It is precisely because of the comprehensive prevention and treatment strategy based on hepatitis B vaccination. The epidemiological survey in 2014 showed that the carrier rate of hepatitis B surface antigen in children under 14 years old in China has been less than 1%.
The positive rate of anti-HCV in the general population in China was 0.43%. However, the public is not fully aware of hepatitis C. The long-term prognosis of non-specialists for chronic hepatitis C infection and the effectiveness of antiviral therapy are not fully understood. In April 2017, the State Food and Drug Administration approved the first direct antiviral drug for hepatitis C. The domestically produced direct antiviral drugs for hepatitis C are also coming soon, which will completely change the current status of hepatitis C treatment in China.
In addition, based on the latest developments at home and abroad, the Chinese Medical Association Liver Diseases Branch and the Infectious Diseases Branch have repeatedly developed and updated guidelines for the prevention and treatment of viral hepatitis, providing important technical guidance documents for the standardized treatment of hepatitis.
Despite the great success of hepatitis B vaccine immunization prevention and anti-viral treatment of hepatitis C, the WHO report shows that viral hepatitis remains a major global health problem. According to information released by WHO, as of the end of 2015, there are still 325 million people worldwide suffering from chronic hepatitis; in low- and middle-income countries, infected people are rarely tested and treated; viral hepatitis caused 1.34 million in 2015 Death, which is equivalent to the number of deaths from tuberculosis, exceeds the number of HIV deaths; new cases of hepatitis continue to occur, mainly hepatitis C. The World Health Assembly in May 2016 adopted the “Global Health Sector Viral Hepatitis Strategy 2016-2021†for the first time, emphasizing that eliminating viral hepatitis is not only part of the global health business, but also an integral part of the world's sustainable development goals. Part of it. To this end, the WHO called on the world to act and put forward "to achieve the full elimination of the health threats caused by chronic hepatitis B/C hepatitis by 2030." The specific goal is to reduce the number of new viral hepatitis infections by 90% and the number of viral hepatitis deaths by 65% ​​by 2030. This is a global strategic goal for the prevention and treatment of viral hepatitis. This goal is not so high, because despite the many challenges, global efforts to eliminate hepatitis are making progress.
| Pan Xiaoben |
Pan Xiaoben, MD, researcher at the Institute of Liver Diseases, Peking University, master's tutor. Postdoctoral fellow at the Baruch Blumberg Institute in the United States. The main research direction is the molecular biology and antiviral mechanism of hepatitis B virus.
Source of work: "Intellectuals" (WeChat: The-Intellectual)
"Intellectuals" is a new mobile media platform founded by three scholars, Rao Yi, Lu Bai and Xie Yu. It is dedicated to science, humanities and thought.
The personalized Keychain always popular life,because you can see them in many different place,such as scenic spot,shop,sinema and others.And the custom logo keychains can be made to be lovely and cute.So most people like them very much.Using vibrant colors, kinds of finishes, and durable plating in gold, silver, copper, gun black,rose gold and so on for you choose.
Keychain Custom Logo,Custom Made Keychains,Keychain Metal,Logo Keychains,Custom Made Keychains
Shenzhen MingFengXing Art & Craft Products CO., LTD. , https://www.mf-gift.com